Reports
Health
Wellness
Around 6.2 million Americans of 65 years and above are ravaged by Alzheimer's. Alzheimer's is characterized by amyloid plaques in the brain. A new study found that people taking certain drugs for type 2 diabetes had less amyloid protein in the brain. Further, people taking these drugs also displayed a slower cognitive decline.
Alzheimer’s is one of the ten leading causes of death in the US. Medically, Alzheimer’s is a progressive neurological disorder, i.e., the nerve cells in the brain start to die, and the brain shrinks.
The area of the brain to get affected earliest is the hippocampus, which is responsible for memory. However, the onset of disease can occur much earlier than the appearance of the first symptoms.
Gradually, neuronal cell death progresses to other areas of the brain. This leads to severe memory impairment and loss of ability to carry out everyday tasks.
To date, there is no cure or treatment for Alzheimer’s. Further progression of the disease ultimately results in death due to severe loss of brain function involving dehydration, malnutrition, or infection.
Xcode Life’s Gene Health Report analyzes 50+ genetic markers for Alzheimer’s disease to give possible predisposition and recommendations. Check your Alzheimer’s Disease risk here.
Biological markers or biomarkers are characteristics that can be objectively measured as an indicator of a pathological or normal physical process.
For Alzheimer’s, scientists usually look for two proteins as the disease’s biomarkers.
Amyloid plaques are stacked forms of the beta-amyloid protein fragment. Beta-amyloid is a protein fragment cut from the amyloid protein precursor (APP). Usually, these protein fragments are cleansed from the brain by microglia.
Image Source: Brain Blogger
The image here depicts amyloid plaques formed around nerve cells in the brain.
In Alzheimer's patients, the beta-amyloid does not get eliminated and starts forming clusters in the brain. In their early cluster stage, the beta-amyloid starts destroying synapses or nerve junctions - leading to memory loss in the individual. Upon forming plaques, the beta-amyloid protein contributes towards brain/nerve cell death.
Tau proteins are part of the neuron’s (nerve cell) internal support and transport system.
Image Source: Utah Public Radio
In Alzheimer’s, the tau proteins change their shape and structure to form tangles in the neuronal fibers. These tangles disrupt normal tau protein functioning and become toxic for the cells, thus leading to cell death.
The most prevalent genetic risk factor for Alzheimer’s is the ApoE (apolipoprotein E) gene. The 4 type of this gene is known to confer the highest risk factor and is present among 50% of Alzheimer’s patients.
The ApoE gene present on chromosome 19 makes a protein that helps transport cholesterol and other fat molecules through the bloodstream.
While there are two other types of the ApoE gene ( 2 & 3), only the 4 variant is associated with increased risk for Alzheimer’s. Having one or both copies of ApoE 4 in the body increases Alzheimer’s risk. The prevalence of individuals carrying one copy is about 25%, while only 2-3% carry both copies.
Know your ApoE gene Status with Xcode Life’s Gene Health Report.
Alzheimer’s is one of the diseases where age, especially old age, plays a significant role. Although Alzheimer’s development is not part of the normal aging process, old age increases the risk.
MCI is characterized by a decline in memory and associated thinking abilities, disrupting an individual's normal societal or work-environment functioning. Usually, an MCI diagnosis with primary memory deficit leads to Alzheimer's associated dementia.
Certain factors which pose a risk for cardiac problems also increase Alzheimer’s risk. Some of them are
Additionally, people with type 2 diabetes are at a higher risk of Alzheimer's disease. This may be due to higher blood sugar levels which have been linked to amyloid plaque buildup.
DPP-4 inhibitors or gliptins are oral diabetes drugs used to block the enzyme dipeptidyl peptidase-4. DPP-4i acts on incretins (a group of hormones that stimulate the release of insulin). In addition, it reduces glucagon (a hormone that increases blood sugar levels), thereby decreasing blood sugar levels.
A previous study exploring the effect of DPP-4i use on dementia among type 2 diabetes patients revealed an increased impact on dementia, albeit not in Alzheimer’s patients.
Studies revealed an increased risk of inflammatory bowel and hypoglycemia when combined with another class of diabetic drug, sulphonylureas (like glipizide and glimepiride), in type 2 diabetic patients.
Know your body’s predisposition to the metabolism of DPP-4i and sulphonylurea drugs with Xcode Life’s pharmacogenomics report, Personalized Medicine.
Scientists at the American Academy of Neurology explored the effect of DPP-4i use in Alzheimer’s patients who may/may not suffer from type 2 diabetes (T2D).
The study involved 282 people with either pre-clinical, early, or probable diagnosis of Alzheimer's. Individuals were of an average age of 76 and were followed for a six-year period. These people comprised of:
Researchers measured the amyloid content in the individuals’ brains using a brain scan.
Study participants were made to take a common thinking and memory test called Mini-Mental State Exam (MSME) every 12 months for 2.5 years to track cognitive decline. The test consisted of questions like counting backward from 100 by sevens or copying a picture on paper. The score ranged from zero to thirty.
Between the three subgroups, Alzheimer’s individuals having T2D and on DPP-4i drugs:
Further adjustment of factors that could affect MSME scores, the same Alzheimer’s individuals with T2D and using DPP-4i drugs scored even lower decline by 0.77 points per year.
Zinc is an essential nutrient that plays many important roles in the body. It is ‘essential’ because the body cannot produce zinc on its own, and thus, it should be obtained through food sources.
After iron, zinc is the most abundant trace mineral (minerals required in small quantities) in the body.
Zinc boosts the immune system and is important for metabolic function.
It is well known for its role in wound healing and the sense of taste and smell.
It is a part of many enzymes that are required for sending messages across cells in the body.
Zinc absorption from the diet depends on the total amount of zinc present in the food.
It has been found that the more the amount of zinc present in food, the lower the amount absorbed.
This means that zinc is better absorbed when taken in small doses.
Zinc plays an important role in the functioning of immune cells. So a deficiency in this nutrient can lead to a weakened immune response.
Zinc is present in the part of the cell where the formation of DNA and proteins occur. Protein production from DNA is a multi-step process, where zinc plays an important role in each step.
Gene expression is the process where the information in the gene is used to produce proteins and other gene products. Zinc plays a role in regulating how much protein or product is produced by the genes.
Zinc plays a role in the activity of more than 300 enzymes. The ‘zinc-binding’ sites help one compound attach to another in chemical reactions.
Zinc supports normal growth and development during pregnancy, infancy, and adolescence. According to a study, infants with low birth weight saw significant weight gain improvements when supplemented with zinc.
Zinc has anti-inflammatory properties - the ability to reduce inflammation or swelling. So, it can help with skin problems like acne and rashes.
Zinc is available as supplements in various forms, each of which impacts health in different ways.
Zinc sulfate is the least expensive form available; however, it is also the least absorbed by the body. This form is used for acne treatment.
Other forms of zinc include:
Though the importance of zinc in humans was established only in the 1960s, its impact on agricultural production was identified in 1869 itself, when zinc was reported as an important nutrient for the growth of a fungus, Aspergillus niger. In 1914, it was discovered that maize, a common crop, also required zinc for normal growth. By the 1920s, it was established that zinc is needed for the growth of all higher plants.
The years from 1920 to 1950 witnessed the essentiality of zinc in mice, poultry, and swine. However, researchers were still skeptical about the possibility of zinc deficiency in humans. This ended when the first case of zinc-deficiency-induced dwarfism that resulted in delayed sexual maturation was reported in the United States. Subsequent zinc supplementation resulted in improved growth and development.
In 1974, the National Research Council of the National Academy of Sciences declared zinc as an essential element for humans, and in 1978, FDA mandated the inclusion of zinc in prenatal supplements.
In the developing world, nearly 2 billion people may be affected by zinc deficiency. Consumption of cereal proteins high in phytate was identified as the major culprit for this. Phytate/phytic acid is a natural substance found in plant seeds. It is known for impairing the absorption of various minerals like iron and zinc.
The recommended daily intake (RDI) for adults varies between 8 to 11mg. The maximum tolerable amount is 40mg per day.
Several proteins, called the zinc transporters are responsible for the circulation and absorption of zinc in the body. Zinc homeostasis (ability to maintain stable levels of zinc) is managed by zinc intake and output transporters that are coded by SLC30A and SLC39A gene families.
SLC39A4
SLC39A4 codes for zinc transporter ZIP4, which is responsible for the absorption of zinc in the intestines. Differences in the SLC39A4 can alter the structure of the ZIP4 protein and hence affect zinc absorption. Certain types of SLC39A4 gene are associated with lower zinc levels.
SLC30A2
SLC30A2 codes for zinc transporter 2 or the ZNT2 protein. This gene plays a role in neonatal (newborn) zinc deficiency. A type of this gene produces an ‘incomplete’ ZNT2 protein that results in the poor secretion of zinc into the breast milk. Infants that feed on this zinc-deficient breast milk go on to develop zinc deficiency in their later years. Two SNPs of SLC30A2, rs35235055 - also known as c.68T>C - and rs35623192 - also known as c.1018C>T - play a role in lower zinc secretion in breast milk.
SLC30A8
SLC30A8 codes for zinc transporter 8 or the ZNT8 protein. This protein is responsible for transporting zinc inside insulin cells, thereby promoting insulin release. Differences in the SLC30A8 can affect zinc transport. A certain type of this gene plays a role in increasing the risk of diabetes by reducing zinc transport and decreasing insulin secretion. A study on this gene also concluded that zinc supplementation could fix the error in glucose breakdown (by promoting insulin secretion), thereby treating diabetes.
SLC30A8 SNP rs13266634 is associated with the risk of type 2 diabetes. The CC type was found to have the lowest concentrations of zinc. Further, it was noted that zinc supplementation in people having the C type reduced the blood sugar levels.
The same study claimed that “Zinc intake has a stronger inverse association with fasting glucose concentration in individuals carrying the glucose-raising A allele of another SNP rs11558471 (in SLC30A8 gene.)” This meant that as zinc intake increased, a reduction in blood glucose levels was seen.
SLC30A3SLC30A3 codes for zinc transporter 3 or the ZNT3 protein. This protein is required for the transport of zinc into synapses, which are the site of electronic signalling between two nerve cells.
rs11126936 is an SNP in the SLC30A3 gene. A study found that individuals having TT and TG types had higher levels of zinc levels than those with GG.Previously, another SNP rs73924411 in the same gene was found to play a role in regulating zinc levels in people with cognitive impairment.
IL6
White blood cells express cytokines. Cytokines are a group of proteins that are expressed by the immune system. They play an important role in cellular communication, especially during immune responses.
Some of these cytokines are termed as interleukins - abbreviated as IL. IL6 or interleukin 6 is a cytokine that is produced at the site of inflammation. The IL6 gene encodes IL6 protein. This is mainly responsible for the acute phase response (a response that is raised immediately after an injury/infection).
It also has an anti-inflammatory myokine role. Myokine responses are essentially cytokine responses that occur due to muscle contraction.
The levels of IL6 are related to the zinc levels in our bodies.
The relationship between the IL6 gene and zinc levels is reciprocal.
When there’s a zinc deficiency, it affects the IL6 gene, increasing IL6 cytokine production, which lowers zinc levels even further.
rs1800795
Also known as 174 G/C, rs1800795 affects the zinc levels upon dietary consumption of zinc. A study on the European population revealed that people having the GG type of rs1800975 had higher levels of IL6 (and hence, lower levels of zinc) than those with CC type.
According to the World Health Organization (WHO), about one-third of the world’s population suffers from zinc deficiency.
The tolerable upper intake level (UL) - the maximum amount of nutrient intake that is likely to be not risky for health - for zinc is 40 mg per day for healthy adults. Excessive intake of zinc can lead to zinc toxicity.
Although there are no reported zinc toxicity cases from food sources, some zinc supplementation at incorrect doses could cause a problem.
Zinc levels can be determined using a simple blood plasma test or a urine and hair analysis, since zinc is distributed throughout the body.
However, it is difficult to identify zinc levels using laboratory tests alone.
Doctors may assess other risk factors, including genetics and dietary intake, along with blood test results to identify your zinc requirements.
Zinc is important for the growth and development of immune cells, namely the T-cells and B-cells. It also plays a role in immune responses that require antibody production.
Zinc ions exhibit antimicrobial activity and are necessary for the functioning of natural killer cells (another type of immune cells)
Acrodermatitis enteropathica, a rare disease, is associated with zinc deficiency. This condition increases the risk of viral, fungal, and bacterial infections.
The zinc requirements for women increase during pregnancy. Its deficiency can be harmful to the growing fetus.
A study conducted on mice showed that gestational (during pregnancy) zinc deficiency affected the offsprings' immune function, which persisted for three generations.
Zinc helps the formation of cells that are required for bone building. It also slows down the excessive degradation of bones.
Zinc forms a part of many enzymes that are necessary to hold the structure of bones in place.
According to a study, excess zinc excretion plays a role in the development of osteoporosis.
Hair loss in patches is often seen in people with zinc deficiency. This is because zinc plays an important role in a process that leads to the formation of hair follicles.
Collagen is an important protein that gives structure to the skin and protects it against different strains. Zinc is a crucial component of collagenases, the enzymes that form collagen. According to a study, zinc supplementation can help slow down the degradation of collagen.
Since zinc boosts immune function, it also helps prevent infection in older people. In fact, according to a study, people with adequate zinc levels had a 50% lesser risk of developing pneumonia compared to those who had lower levels.
Zinc deficiency could also occur in people with the following conditions:
Zinc is not stored in the body, so it must be included in the diet to ensure sufficient amounts are available. A healthy and balanced diet, which includes zinc-rich foods, will ensure sufficient vitamin and nutrient intake.
Zinc is a trace mineral that is important for its role in immune function, growth and development, and protein production. The role of zinc in human health was only identified in the 1960s, and since then, the FDA has made it mandatory to include it in all prenatal products. The SLC gene family codes for proteins that are responsible for zinc transport and absorption in the body. Studies have shown that rs13266634 in the SLC30A8 gene plays a role in zinc transport into insulin cells. Individuals who have the CC type have decreased transport of zinc to the insulin cells. This results in lowered secretion of insulin, and hence a higher risk for type 2 diabetes. rs35235055 and rs35623192 are two SNPs in SLC30A2 gene that are important for transport of zinc to the breast milk. Lower levels of zinc in breast milk can increase the risk for neonatal zinc deficiency. The recommended daily intake (RDI) for adults varies between 8 to 11mg, with maximum tolerable amount being 40mg per day. Oysters are an excellent source of zinc with one serving providing over 600% of the RDI. Some plant based food sources rich in zinc are tofu, legumes, hemp seeds, and nuts.
Regardless of what your fitness goals may be, aerobic capacity is an important metric to focus on. It determines how well your body can utilize oxygen. Simply put, the better the aerobic capacity, the longer you will be able to sustain exercises. Aerobic training not only helps achieve peak fitness but also improves cardiac health and respiratory functions. An individual's genetic makeup can determine up to 50% of their aerobic capacity by influencing factors such as antioxidant production, heart functioning, etc. The analysis of such genes and their variants can give a clearer idea of the kind of training you need to take on to achieve maximum results.
Aerobic capacity (AC) is the maximum amount of oxygen consumed while performing intense activities that involve large muscle groups.
It is also a measure of how effectively the heart and the lungs get oxygen to the muscles. Hence, improving your aerobic capacity can directly result in more efficient use of oxygen by the body.
The other term which is used to describe aerobic capacity is vO2 max.
However, the vO2 max also takes into consideration the individual's body weight.
One of the best ways to estimate your cardiovascular fitness is by calculating your Aerobic Capacity.
If you are in a fitness center, one of the following two techniques can be used to measure your AC
A simpler and less accurate way of measurement is a walk/run test.
This requires walking/running at the maximum speed you can and measuring your heart rate at the end of it.
With this measurement, you can use one of the many online calculators that are available to check your Aerobic Capacity.
For instance, Rockport walk test is one such calculator that requires the input of your heart rate, time of the run/walk, and your weight to calculate your Aerobic Capacity.
Genes majorly control a lot of factors that have an association with the fitness levels of an individual.
According to a study in 2016, 155 genetic markers were found to be associated with better athletic performance, 93 of which were endurance-related markers, and the other 62 were power/strength related markers.
Polymorphisms of ACE, ADRB, ACTN3, PPARGC1A were one of the first genetic markers found to be associated with athletic performance.
There's another famous exercise genetics study conducted by a consortium of five universities in the United States and Canada revealed astonishing variation in the aerobic capacity amongst the 481 participants.
The study subjected its participants to identical stationary-bicycle training regimens with three workouts per week of increasing intensity under strict control in the lab.
The results
These can be attributed to the variants of genes like NRF1, NRF2, VEGF, PPARA, etc. that an individual carries.
The nuclear respiratory factor (NRF2) gene influences the vo2 max. NRF2 regulates the expression of antioxidant proteins and thus can influence the oxygen uptake.
| Genotype | Implication |
|---|---|
| AA | 57.5 % higher training response |
| CC | Normal training response |
Some genes affect a few secondary traits that exert influence on aerobic capacity.
For example, genetic variations in VEGF in the gene influence heart structure, size, and function. These have an impact on the stroke volume which is an important determinant of aerobic performance.
| Genotype | Implication |
|---|---|
| GG | Reduced aerobic performance |
| CC | Normal aerobic performance |
Genetics is only 50% of the fitness story.
The rest wires down to other factors like your lifestyle, your eating habits, and your training.
Getting at least 150 minutes of moderate aerobic exercise, or 75 minutes of vigorous activity each week is vital to ensure a longer healthier life
Augmenting your aerobic capacity can result in better blood and oxygen flow to muscles.
Therefore, this promotes faster recovery between sets and improves your flexibility.
Aerobic exercises include walking, running, cycling, swimming, and almost every other cardio workout.
When aerobic exercises are performed, your heart is trained to deliver more oxygen in a said span of time, and at the same time, your muscles are trained to utilize the oxygen delivered more efficiently.
To improve your aerobic capacity, it is important to understand how your body builds endurance.
It depends on the following three things:
When you train to increase all the above-mentioned variables, naturally the amount of blood and oxygen, reaching your muscles increase.
This, in turn, has a positive effect on your overall athletic performance.
Aerobic training usually, targets large muscle groups of your body that boost your heart rate for longer periods of time.
Some of the commonly recommended aerobic exercises include
Some of the aerobic exercises that you can do at home include:
If you are already not inspired to take up aerobics, take a look at the benefits you can acquire from aerobic training.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4114002/
https://www.ncbi.nlm.nih.gov/pubmed/17357964
https://www.ncbi.nlm.nih.gov/pubmed/25729143
Upload your raw data to Xcode Life for insights into 700+ health-related traits!
Ever wondered how going off carbs for a couple of days almost always shows instant results? Thank all the glycogen stored in your body. But some people have trouble storing this entity as we do, disrupting their chance to live a typical life. Let’s peak into what actually happens with Glycogen Storage Disease.
Metabolism is a biological process that breaks down the food we eat and provides energy to keep us alive.
In some cases, different key stakeholders in this process fail to fulfill their duty.
There is a spectrum of conditions that fall under the umbrella of Glycogen Storage Disease (GSD), which cause such trouble.
Since it did not have any popular awareness challenge go viral (bring back the ice buckets!) and fortunately is a rare condition, it hasn’t had too much light thrown on it.
According to the reports, the incidence rates appear to be 1 in 100,000.
As the symptoms set out at an early age, Glycogen Storage Disease appears to affect the little ones more than the adults.

Our body uses glycogen, a complex sugar compound, as a fundamental storing unit of energy. Metabolizing glycogen, to break it down into glucose, provides the instant energy we need. As we need to conserve some of this energy, these glucose molecules are combined back into glycogen. This is used as a reservoir to tap into when there is a lack of energy supply. Different parts of the muscles and the liver act as storage units for glycogen.
For this process to take place, some special proteins called enzymes (biocatalysts) aid the formation and deformation of glycogen.
When these enzymes don't function optimally, it leads to a spectrum of diseases.
This could lead to a range of different symptoms depending on the type of diseases.
The types are classified based on the enzyme which is at fault. It starts from GSD1 and runs up until GSD 15.
The types 1 to 4 cover almost 90% of the reported cases, with sub-type of GSD1 - GSD 1a, aka Von Gierke Disease being most common.
As the condition affects the necessity of food metabolism, symptoms start to show 3 to 4 months after birth.
The cause of Glycogen Storage Disease is genetic.
The gene that is responsible for the malfunction of the enzyme can pass on from the parent generation to the next, making it hereditary.
But the child will express symptoms of the associated GSD, only when both parents possess a defective gene.
Every human gets their 23 chromosomes from each parent.
Genes that are subunits to chromosomes have one trait, which is dominant and other recessive (some exceptions, of course).
The level of expression marks the difference between them.
Now, for the expression of a recessive trait, both parents must pass on their recessive versions of traits.
The Mendelian world calls this an autosomal recessive condition.
A random process selects the gene to be passed down to the next generation.
In the case of autosomal recessive conditions, there is a 25% chance of occurrence.
If just one parent passes it on, then the child will remain healthy but acts as a carrier of the gene.
Carriers could potentially pass it on to the coming generations.
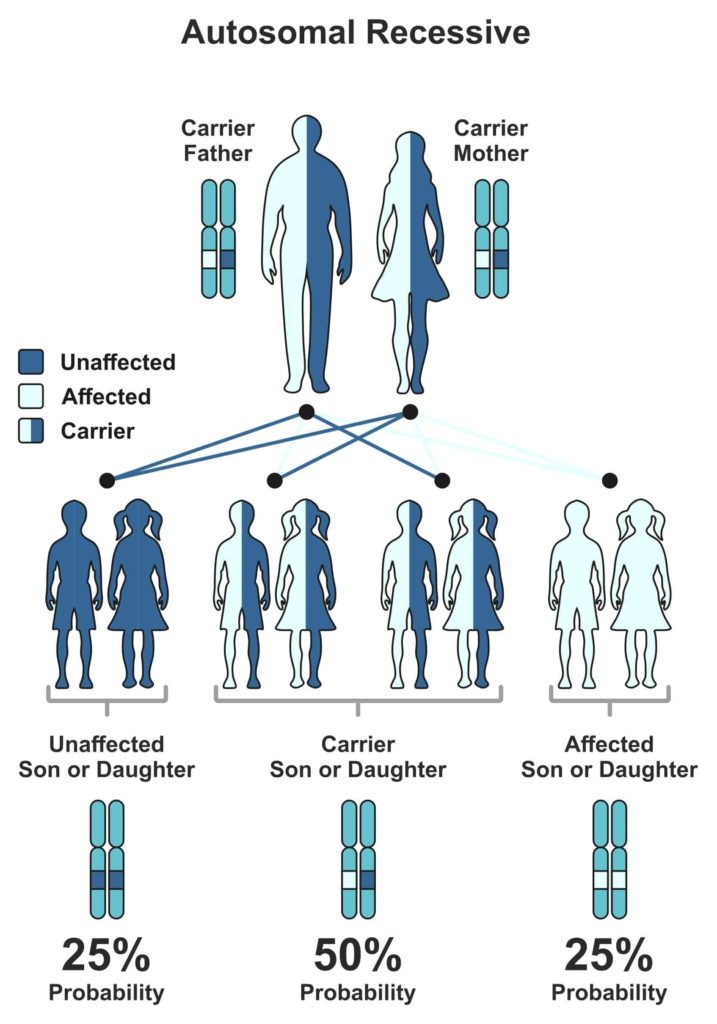
We obtain glucose from the diet we eat.
Glycogenin is an enzyme that is responsible for cutting down this glucose into short fragments.
Another enzyme, glycogen synthase, helps in the conversion of glucose into Glycogen.
Now, some branching enzymes add branches to Glycogen, which the liver then stores as a reservoir of energy.
When we fast or there is a need for muscle contraction, the body taps into the said reservoir.
The process of breakdown involves four enzymes.
Glycogen Phosphorylase and glycogen debranching enzymes help in unraveling the molecule to release glucose and expend energy.
Another method of breaking it down involves enzymes such as a-glucosidase and Glucose-6-phosphatase.
Every action in our body is instructed by the beautifully wound helix, DNA.
As these enzymes correspond to specific genes, any defect in this gene will directly translate into the enzyme’s action.
Take the example of the most common type of GSD - GSD type 1a.
One of the enzyme genes involved in breaking down glycogen is Glucose-6-phosphatase. G6PC and SLC37A4 genes code for this enzyme.
As genes code for proteins and proteins like enzymes carry out the function, an error in the gene structure or function will lead to a collapse of the entire system.
A good analogy would be a loose brick in a building that could make it fall into pieces. Geneticists call these errors, mutations. As these ‘errors’ have been major contributors in the history of evolution, they have been beneficial in many ways.
Unfortunately, not in the case of Glycogen Storage Diseases.
So, when there is a mutation in this gene, glucose-6-phosphatase does not play its role and leads to the build-up of Glycogen and fat.
We know, too much of anything is toxic. Hence, the accumulation of Glycogen and fat hinders the function of organs like liver and kidney.
In the case of type 3 Cori disease/Forbes disease, a distinct part of the gene, called Exon3, carries two mutations that cause the debranching enzyme to malfunction.
As a debranching enzyme is responsible for the decomposition of Glycogen, it leads to toxic accumulation.
Andersen disease (type 4) affects the GBE1 gene that codes for glycogen branching enzyme leading to large amounts of abnormal Glycogen accumulated, causing severe conditions like liver cirrhosis, which is ‘doctor’ for scarring.
Types 1, 3, and 4 are far more common in comparison to other types.
Most often, all different types have a combination of some common symptoms in varying intensities.
As the age of onset is rather young in the case of Glycogen Storage Diseases, doctors generally ask the parents about symptoms showcased by their child and call for relevant tests.
Blood tests and MRI/ultrasound scans are routine. In some cases, a biopsy of a suspected organ might be required for confirmatory diagnosis.
Genetic testing has evolved over time and is performed to confirm the diagnosis.
This is extremely helpful in the case of couples who have observed a family history of Glycogen Storage Disease.
Seeking genetic counseling before planning a family will help provide a clearer picture of the chance of occurrence in their case.
Carrier testing for at-risk family members and prenatal diagnosis have led to significant changes in family planning.
The treatment options are specific to the type of GSD diagnosed with the patient.
Generally, it includes major dietary restrictions.
Uncooked corn starch can be a good feed to children over two years of age, as corn starch can promise a slow release of glucose.
This is fed in small portions throughout the day.
For type 1, elimination of food which is high in lactose and fructose is advised (that's pretty much everything tasty, from mozzarella to maple syrup).
Allopurinol is prescribed if there is a risk of kidney stones or gout, as it reduces the levels of uric acid in the blood.
Some extreme cases like the type 4 of GSD could require liver transplant depending on the extent of the condition.
When the patient is prone to frequent muscle cramps during exercise, a high protein diet is advised.
In some cases, the intake of glucose and fructose is advised. And of course, as their muscles are now more susceptible to damage, over-exhausting the body is a big No-No.
Unfortunately, prevention is barely an option.
As this is a genetic condition, the only way of prevention is to ‘not pass on the genes’.
Parents can seek genetic counseling to check if both carry the defective gene as it takes two to tango in this case.
But we have come a long way with the prognosis of the condition.
The unawareness of the disease’s details led medical researchers to believe GSD to be a fatal condition until the early ’70s.
People now live long and normal lives with significant dietary shifts.
This barely seems like a change in the millennial world of Keto and Atkins!
Speaking of Keto and Atkins, Glycogen is the secret quarterback in the low-carb diet game.
Glycogen storage and weight loss have a strange love story.
A typical healthy liver in an individual can hold up to 400 grams of glycogen and muscle cells, about 100 grams.
As glycogen and water co-exist in a 1:3 ratio, there are 3 grams of water present for every gram of glycogen.
This is the real reason behind the initial weight loss observed when any sort of low-carb diet is practiced.
It would rather be ideal for comparing body fat percentage or keeping track of monthly weight changes, for getting a better measure of body change as opposed to this initial quick loss of weight.
The body stores about 1500-2000 calories of Glycogen typically.
With a low-carb diet, the body uses this up and has little or no reservoir of energy. Possibly dehydrated.
This can lead to a constant state of fatigue in some extreme cases that could potentially damage the liver as it would be exposed to undue stress.
So we should be cautious when we make such lifestyle changes and always seek professional guidance.
In the case of sportsmen, a low-carb diet could lead to quick use of the stored Glycogen, especially the ones stored in the muscles.
This leads to a case of muscle fatigue and, in extreme cases, will lead to a phenomenon known as “hitting the wall”. Glycogen is also brain food.
So severe lack of Glycogen leads to cognitive symptoms like confusion, disorientation when you have a “bonk” during exercise.
Though there is cutting current edge research to discover new enzyme replacement therapies and gene therapies, this spectrum of diseases affects many lives.
But once again, we are hopeful that genomics will save the day!
Upload your DNA raw data to Xcode Life for insights into 700+ health-related traits
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4146814/ - Ozen H. (2007). Glycogen storage diseases: new perspectives. World journal of gastroenterology, 13(18), 2541–2553. https://doi.org/10.3748/wjg.v13.i18.2541
https://www.tandfonline.com/doi/full/10.3109/01913123.2011.601404 - Hicks J, Wartchow E, Mierau G. Glycogen storage diseases: a brief review and update on clinical features, genetic abnormalities, pathologic features, and treatment. Ultrastruct Pathol. 2011;35(5):183‐196. doi:10.3109/01913123.2011.601404
https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(04)16986-9/fulltext Astrup, A., Meinert Larsen, T., & Harper, A. (2004). Atkins and other low-carbohydrate diets: hoax or an effective tool for weight loss?. Lancet (London, England), 364(9437), 897–899. https://doi.org/10.1016/S0140-6736(04)16986-9
https://onlinelibrary.wiley.com/doi/abs/10.1038/icb.2015.109 - Gleeson M. (2016). Immunological aspects of sport nutrition. Immunology and cell biology, 94(2), 117–123. https://doi.org/10.1038/icb.2015.109
Carbohydrates are one of the main classes of food. It is the main source of energy for the body. They are a group of organic compounds present in the form of food in cellulose, starch, and sugar. They are called carbohydrates, as they contain hydrogen and oxygen in the same ratio as water (2:1). These are then broken down to release energy.
Due to the association of carbohydrates with weight gain, the carbs may disguise as an enemy to a healthy diet. However, the right kind of carbs in the right amounts can definitely earn a rightful place in your diet.
A healthy weight is an important element of good health. The amount of food you eat and what you eat is important for maintaining a healthy weight.
For years, there was a myth that a low-carb diet is the best way to lose weight, but a growing body of evidence suggests otherwise.
Carbs are sub-divided into three categories depending on the number of sugars present and the nature of the chemical bonds between them.
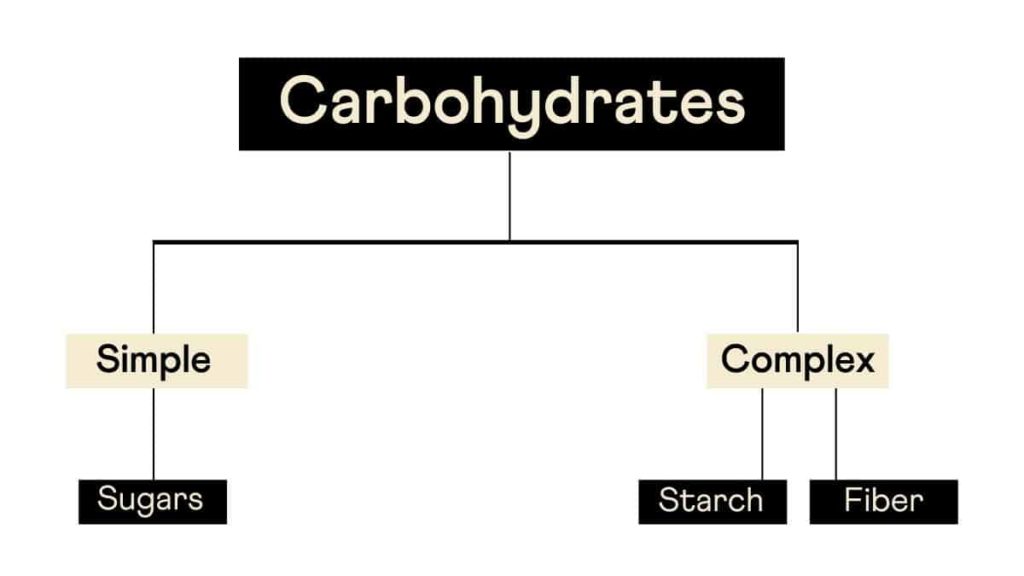
Although this is the conventional way of classifying carbs, a more pragmatic approach would be to classify them as refined and whole carbs.
Whole carbs include vegetables, legumes, whole fruits, and grains, which are unprocessed and thus have their nutrient content intact.
The stripping of nutrients in refined carbs as a part of processing makes them count as 'empty calories.'
This removal of the nutrients results in rapid absorption and metabolism of these carbohydrates.
This results in spiked sugar levels and unstable energy levels, the latter of which causes “sugar rush” after consuming sugar-rich foods.
The refined or the sugary carbs are simple carbohydrates that the body quickly absorbs.
Their metabolism occurs rapidly, which results in major swings in the blood glucose levels. This induces hormonal and metabolic changes that can promote overeating.
Not all carbohydrates are bad for health.
Unprocessed carbs that are present in vegetables, fruits, and grains are healthy.
Studies reveal that these improve metabolization and help in weight loss.
Another class of carbs, the processed ones, are unhealthy because they lose the fiber during processing and contain no essential nutrients.
Examples of these include white bread, white rice, and the like.
Intake of foods containing processed carbs can result in weight gain and increased risk of type 2 diabetes.
Studies show that high unprocessed carbs in the diet can lead to a craving for more carbs, and people tend to get caught in the vicious cycle.
Thus, making changes to your diet by including more of unprocessed carbs can be a healthy choice.
Studies have shown that there is a link between genetic factors and dietary factors such as weight loss, weight gain, obesity.
Nearly 70% of the world population carries the gene for obesity. This explains why obesity is growing rapidly around the world.
However, people can reverse this gene's effect by exercising and including more protein in their diet.
A study has found an association between the FTO gene and the intake of carbohydrates.
The individuals with the A variant were found to have a higher risk of obesity than ones with the CC wild type.
The FTO gene has a negative association with over-eating.
About 23% of the global population carries a variant in the AMY1 gene, which shows an association with low copy numbers of the gene and reduced ability to digest starch.
70% of people from agricultural populations have an AMY1 copy number variant, which shows an association with better starch digestion and lower risk of obesity when compared to 37% of non-agricultural populations.
The conventional diet plans and workout regimes architected for weight loss need not help everyone achieve their desired goals.
There is a multitude of factors that influence weight loss, including a person’s lifestyle, genetic makeup, and the environment.
Your genes can influence how you metabolize the nutrients you get from your diet. This directly has a role to play in weight gain/loss.
For instance, some people may possess a genetic variant that aids in the faster metabolization of carbohydrates, while others may carry a variant that will help in faster break-down of saturated fats.
You can leverage such genetic information to adopt a practical and personalized weight loss plan.
While genetic tests may not lay out the A-Z of weight loss, it certainly helps you pin the right path for your weight loss journey.
Upload your DNA raw data to Xcode Life for insights into 700+ health-related traits
The Fat Mass and Obesity gene (FTO) is associated with the synthesis of the FTO enzyme, also known as the alpha ketoglutarate dependent dioxygenase. FTO gene expression has been shown to be upregulated in the hypothalamus of the brain after food deprivation, which is associated with increased consumption of high calorie foods. There are 3 SNPs that we analyze in association with weight management and carbohydrates, fiber, protein or saturated fat intake- rs9939609, rs8050136 and rs11076023.
In a review study, children with the A variant of the gene were shown to be associated with greater intake of biscuits when compared with children with the T variant (rs9939609). Adults with the A variant (rs9939609) were also shown to have altered post prandial satiety (feeling of being full) levels. In a similar study conducted to identify the association between post prandial satiety and rs9939609, people with the A variant of the fat gene were found to be associated with low sensation of feeling full after a meal.
Loss Of Control Over Eating
In a study conducted on 289 youth, people with the A variant (rs9939609) of the gene had a greater tendency to eat more fat than people with the T variant (rs9939609), with 37.4% of people with the A variant reporting loss of control over eating when compared with 18.8% of people with the T variant. Subsequently, the study showed that people with the A variant of the gene had a significantly greater body mass index.
TV advertisement and overeating
TV advertisements could also induce people to overeat, but the extent of overeating is shown to be associated with the fat gene variant carried by the individual. A study was conducted on 200 children post lunch, involving screening of a 34 minute television program, which also included food advertisements and toy advertisements. Children with the A variant (rs9939609) of the gene consumed significantly more snacks when they were exposed to food advertisements.
In a study conducted on 1618 people from an Asian Indian population, people with the A variant (rs8050136) of the gene had a 2.46% higher risk of obesity when compared to people with the C variant among people on a high carbohydrate diet. In the same study, it was found that among people who were inactive, people with the A variant were found to be associated with 1.89 times increased risk for obesity.
In the study on 1618 people from an Asian population, people with the A variant (rs11076023) of the gene were shown to be associated with 1.62cm lower waist circumference than people with the T variant of the gene.
In a study conducted to identify the association between fat intake, FTO genotype (rs9939609) and body fat percentage, people with the T variant did not show an increase in body fat percentage with increase in fat intake, but people with the A variant were shown to be associated with increased body fat percentage with increased fat intake.
In a study conducted on 4839 men and women on a high fat diet, people with the A variant (rs9939609) were shown to be associated with higher BMI and were twice as likely to be obese when compared with people with the T variant.
In a study conducted on 737 overweight adults, people with the A variant of the gene (rs9939609) were shown to be associated with a reduced food craving on a hypo-caloric and a high protein diet.
In a study conducted on 2577 Korean study participants, it was found that people with the A variant (rs9939609) of the fat gene were associated with increased risk for obesity and, thereby, an increased risk for diabetes. In a similar study conducted on 4,189 Han Chinese individuals, people with the A variant of rs9939609 and A variant of rs8050136 were shown to be associated with type 2 diabetes and obesity in the Asian population.
| CHIP Version | FTO SNPs |
| 23andMe (Use your 23andme raw data to know your FTO Variant) | |
| v1 23andme | Present |
| v2 23andme | Present |
| v3 23andme | Present |
| v4 23andme | Present |
| V5 23andme (current chip) | Present |
| AncestryDNA (Use your ancestry DNA raw data to know your FTO Variant) | |
| v1 ancestry DNA | Present |
| V2 ancestry DNA (current chip) | Present |
| Family Tree DNA (Use your FTDNA raw data to know your FTO Variant) | |
| OmniExpress microarray chip | Present |
| Genotype (rs9939609) | Phenotype | Recommendations |
| AA | [Limitation] More likely to have higher risk for obesity. [Limitation] More likely to have higher fat percentage on a high fat diet [Limitation] More likely to have higher BMI on a high fat diet [Limitation] More Likely to have loss of control eating episodes [Limitation] More Likely to have higher risk for type 2 diabetes [Advantage] More likely to have lower food craving on a high protein diet | A common fat gene myth is that people with this variant are doomed to remain overweight or obese, however, practicing mindful eating and consciously avoiding increased consumption of high fatty food could help in better weight management For people with this variant of the fat gene weight loss can be achieved by balancing food intake with appropriate exercises A high protein diet may be beneficial as it reduces food craving. So foods that turn off fat genes are foods that are rich in protein. Spirulina is a rich source of protein and helps improve satiety |
| AT | Moderate risk for obesity | |
| TT | [Advantage] More likely to have lower risk for obesity. [Advantage] Less likely to have higher fat percentage on a high fat diet [Advantage] Less likely to have increased BMI on a high fat diet [Advantage] Less Likely to have loss of control eating episodes [Advantage] Less Likely to have higher risk for type 2 diabetes [Limitation] Less likely to have lower food craving on a high protein diet | Ensure a healthy balance between food intake and exercises |
| Genotype (rs8050136) | Phenotype | Recommendation |
| AA | [Limitation] More likely to have increased risk for obesity. [Limitation] More Likely to have higher risk for type 2 diabetes | Ensure a healthy balance between food intake and exercises |
| AC | Moderate risk for obesity | Though the risk for obesity is lower, practise healthy eating practices and ensure sufficient physical activity |
| CC | [Advantage] More likely to have lower risk for obesity. [Advantage] Less Likely to have higher risk for type 2 diabetes | Though the risk for obesity is lower, practise healthy eating practices and ensure sufficient physical activity |
| Genotype (rs11076023) | Phenotype | Recommendation |
| AA | [Advantage] More likely to have lower waist circumference on a high fiber diet | Consume a fibre rich diet to ensure better weight maintenance Fibre rich foods include black beans, lima beans, broccoli, artichoke and brussels sprouts |
| AT | Moderate waist circumference on a high fiber diet | A fiber rich diet may not considerably lower weight but it helps in bowel movements and in controlling blood sugar levels |
| TT | [Limitation] More likely to have higher waist circumference than people with the A variant on a high fiber diet | A fiber rich diet may not considerably lower weight but it helps in bowel movements and in controlling blood sugar levels |
“Nutrigenetics, fitness genetics, health genetics are all nascent but rapidly growing areas within human genetics. The information provided herein is based on preliminary scientific studies and it is to be read and understood in that context.”
The Tumor Necrosis Factor Alpha (TNFA) gene is associated with the synthesis of TNFA, a pro-inflammatory cytokine that regulates immune responses such as inflammation. Specific alleles of this gene are known to either increase or decrease the levels of TNFA. People with the A variant of the gene are found to synthesize more TNFA which affects fatigue, inflammatory response, diabetes and cardiovascular risk and response to training.
People with the A variant require longer recovery times compared to people with the G variant. A study which was part of the HERITAGE study measured CRP before and after an endurance training programme lasting 20 weeks. The baseline CRP levels were higher among people with the A variant when compared to people with the G Variant. After the training programme, there was a higher increase in CRP among people with the A variant than among people with the G variant. This shows that people with the A variant required longer recovery periods in between intense exercise. A study conducted to identify the association between physical exercise and anti-inflammatory response found that polymorphisms in TNFA gene was associated with the levels of serum C reactive protein after moderate to vigorous physical activity. People with the G variant had a greater decrease in C reactive protein upon physical activity than people with the A variant.
| CHIP Version | TNFA SNPs |
| 23andMe (Use your 23andme raw data to know your TNFA Variant) | |
| v1 23andme | Present |
| v2 23andme | Present |
| v3 23andme | Present |
| v4 23andme | Present |
| V5 23andme (current chip) | Present |
| AncestryDNA (Use your ancestry DNA raw data to know your TNFA Variant) | |
| v1 ancestry DNA | Present |
| V2 ancestry DNA (current chip) | Present |
| Family Tree DNA (Use your FTDNA raw data to know your TNFA Variant) | |
| OmniExpress microarray chip | Present |
In a study conducted on the influence of TNFA gene polymorphisms on fatigue, people with the A variant of the gene were associated with increased sleep disturbance and fatigue than people with the G variant. In another study conducted on elderly women, people with the G variant had a better physical performance independent of their exercise modality.
Association with Cholesterol levels In a study aimed at understanding the metabolic response of people on a high protein/low carbohydrate diet showed that people with the GG genotype had a greater reduction in total cholesterol, triglycerides and LDL cholesterol.
In a study conducted as a part of the Finnish Diabetes Prevention Study, the presence of the A variant was shown to be a predictor for the conversion from Impaired Glucose Tolerance (IGT) to type 2 Diabetes. Another study showed that the presence of the A variant of the gene was associated with an increased risk for diabetes and higher glucose levels in the body.
In a study conducted on postmenopausal women, people with the A variant were associated with increased risk for osteoporosis. Higher levels of cytokines lead to an increase in bone breakdown.
People with the A variant were shown to have an increased risk for acute myocardial infarction.
| Genotype rs1800629 | Phenotype | Recommendation |
| AA | [Limitation] More likely to have higher TNFA level [Limitation] More likely to have higher levels of inflammation after intense exercise [Limitation] More likely to require longer period of recovery [Limitation] More likely to have higher level of fatigue [Limitation] More likely to have higher risk for Diabetes [Limitation] More likely to have higher risk for Myocardial Infarction [Limitation] More likely to have lower reduction in triglyceride, cholesterol levels on a high protein/ low carbohydrate diet | Likely to have lower reduction in inflammation upon exercising than G variants. Increased risk for fatigue post exercises requires optimum periods of rest between repetitions Include 3g of Omega 3 fatty acids in the diet which is found to lower the levels of inflammation |
| AG | Moderate level of C reactive protein and more likely to have moderate level of risk for diabetes | Likely to have lower reduction in inflammation upon exercising than G variants. Increased risk for fatigue post exercises requires optimum periods of rest between repetitions Include 3g of Omega 3 fatty acids in the diet which is found to lower the levels of inflammation |
| GG | [Advantage] More likely to have normal level of TNFA [Advantage] More likely to have lower levels of inflammation after intense exercise [Advantage] More likely to require shorter period of recovery [Advantage] More likely to have lower level of fatigue [Advantage] More likely to have lower risk for Diabetes [Advantage] More likely to have lower risk for Myocardial Infarction [Advantage] More likely to have higher reduction in triglyceride, cholesterol levels on a high protein/ low carbohydrate diet | Likely to have higher reduction in inflammation upon exercising Include Omega 3 fatty acids in the diet |
“Nutrigenetics, fitness genetics, health genetics are all nascent but rapidly growing areas within human genetics. The information provided herein is based on preliminary scientific studies and it is to be read and understood in that context.”
Diabetes is the most challenging health problem of the 21st century with India being the diabetic capital of the world. More than 65 million Indians are affected from type 2 diabetes and the number is expected to double in the next 20 years. The most shocking news is that 270 million Indians are below the poverty line and yet there is an increase in the diabetic peak. Unlike other health conditions, diabetes does not seem to be inherited in a simple pattern, it is twisted and complicated with a combination of genetic make-up and environmental factors. Yet, most people are born with higher susceptibility to develop type 2 diabetes. According to ADA, the risk of developing type 2 diabetes is:
Type 2 diabetes is mainly caused by an interaction between our genetic makeup and our unhealthy lifestyle choices. Type 2 diabetes is a polygenic disorder where numerous genes have been identified as risk factors. The variation in genes confers some impairment in insulin secretion, utilization, and glucose & lipid homeostasis. If you have type 2 diabetes your body does not use insulin properly. This is called insulin resistance. At first, your pancreas will produce extra insulin to make up for it but, over a period of time, it cannot make enough insulin to keep the blood glucose at normal levels.
The root cause of type 2 diabetes is obesity. It runs down the family as they tend to have a similar lifestyle and eating pattern. Obesity is 80% correlated with diabetes. If you have a family history of obesity it becomes difficult to figure out if your diabetes is mainly caused due to lifestyle factors or genetic variations. It is natural that sedentary parents tend to have sedentary children and their unhealthy eating habits are passed on to the next generation. However, studies have shown that in obese individuals, by losing even 4 kg of the body weight, can reduce the risk of developing type 2 diabetes.
Here are 5 alternative healthy foods to knock out type 2 diabetes:
It’s never too late to start caring for yourself and Xcode provides products and services based on metabolic and fitness genotyping. Even if you carry a genetic risk variant, you can still minimize your risk by understanding your genetics and choosing the right food and lifestyle options that are compatible with your genetic makeup. So take action today to prevent and knock out diabetes.
